Dopamine D2 Receptor Downregulation Is the Hallmark of Addiction (Dopamine 4)
- Hermes Solenzol

- Nov 14, 2025
- 11 min read
Updated: Nov 15, 2025
D1 receptors drive desire while D2 receptors mediate self-control

In the three previous articles in this series, I explained the relationship of dopamine with mental energy and addiction. I described the reward pathway and other dopaminergic pathways in the brain. Finally, I showed that ‘dopamine depletion’ at its synapses probably means changes in the dopamine receptors. Therefore, I this article I will describe the five dopamine receptors (D1-D5) and show how they are involved in mental energy and addiction.
How dopamine receptors work
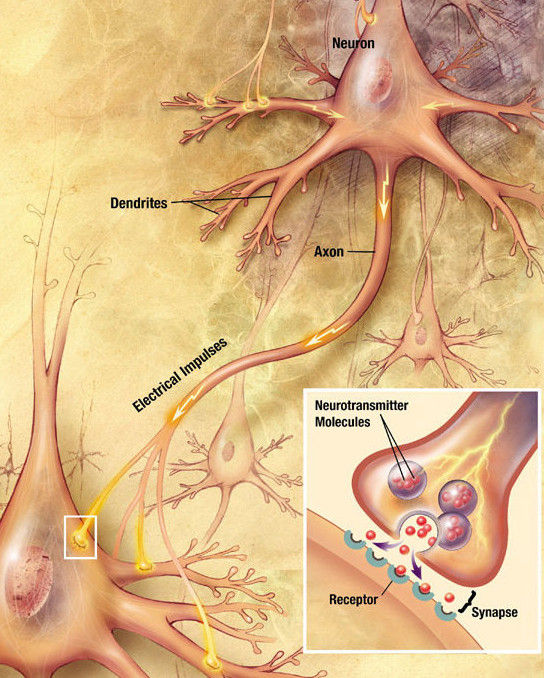
Neurotransmitter receptors are proteins embedded in the membrane of neurons that bind the neurotransmitter. It’s like the neurotransmitter is a key that enters a key lock, the receptor, to open a door. Indeed, there is a pocket in the receptor that fits the neurotransmitter, just like a lock fits a key. And just like a key turns to open the lock, a neurotransmitter changes the conformation of the receptor to send a signal inside the neuron.

Neurotransmitter receptors signal using three main molecular mechanisms:
Some receptors are ion channels: they form a pore across the membrane to allow ions to come in or out of the cell. Ions are atoms with too many or too few electrons, which gives them a negative or a positive electrical charge, respectively. Common positively charged ions (called anions) are like sodium (Na+), potassium (K+) and calcium (Ca2+), whereas chloride (Cl-) is the most common negative ion (cation). These receptors behave much like a door: when they bind the neurotransmitter, their channel opens up, letting through the ions. Each receptor specializes in letting through a particular ion. For example, GABA-A receptors are chloride channels, while glutamate receptors are sodium channels. When ions move through the membrane, they change its electric potential, which changes the ability of the neuron to fire action potentials in its axon. Their signal is fast and short-lasting.
Other receptors signal through G proteins, which attach to their intracellular side. G proteins are formed by three subunits: alpha, beta and gamma (see figure). Alpha subunits act as a bridge to enzymes that produce a second messenger: a small molecule that diffuses inside the cell and changes the function of different proteins. Different alpha subunits, named with lowercase letters like s, i, o, and q, activate or inhibit different enzymes. The beta and gamma subunits send other signals inside the cell. The signal of G protein-coupled receptors (GPCRs) is medium-lasting.
A third type of receptors are enzymes called tyrosine kinases. When the receptor is activated by the neurotransmitter, the tyrosine kinase attaches a phosphate to key proteins, radically altering their function. This changes the behavior of the neuron, making it change, grow or wither. They often trigger major changes in neurons that are long-lasting.
There are five dopamine receptors, named D1 through D5 (Hurley and Jenner, 2006; Mishra et al., 2018). All of them are GPCRs, the second type of receptors. The G proteins to which they are coupled either increase or decrease a key signaling molecule, cyclic adenosine-monophosphate (cAMP). cAMP has many effects, but most of them end up exciting the neuron, that is, making it fire more action potentials.
Internalization and downregulation of receptors
Dopamine receptors are not just present at the synapse, but also all over the membrane of the postsynaptic neuron. This means that dopamine operates by volume transmission: it diffuses away from the synapse, activating receptors in nearby neurons. Some scientists call molecules that act this way neuromodulators, as opposed to neurotransmitters. Other substances that act by volume transmission include neuropeptides like the endorphins, substance P and oxytocin.
When dopamine receptors are activated by dopamine, they cluster at the membrane and then get taken inside the neuron, forming endosomes, a process called receptor internalization (see figure at the top for an example). Internalized receptors cannot be activated by dopamine until they are recycled back to the membrane, a process that takes about 30 minutes. Some of the receptors are not recycled, but destroyed instead.
Repeated internalization results in a decrease of the receptors in the neuron, which is called downregulation of the receptors.
The two families of dopamine receptors
The five dopamine receptors are divided into two families, depending on whether they increase cAMP, and hence excite the neuron, or decrease it, inhibiting the neuron.
The D1 family comprises the D1 and D5 receptors. They couple to an alpha-s G protein (Gs); ‘s’ means stimulatory because it stimulates the synthesis of cAMP. D1 and D5 receptors excite neurons in the nucleus accumbens (the reward pathway) and the frontal cortex (the mesocortical pathway), making them more active and thus increasing motivation.
The D2 family comprises D2, D3 and D4 receptors. They couple to an alpha-i G protein (Gi); ‘i’ means inhibitory because it inhibits adenylyl cyclase, the enzyme that makes cAMP. This inhibits the neurons that have these receptors, so that they fire fewer action potentials. D2 family receptors also couple to alpha-o G proteins, where ‘o’ stands for other (Jiang et al., 2001). The function of Go proteins is still not well understood, but they inhibit some types of adenylyl cyclase, open potassium channels and close calcium channels, resulting in neuronal inhibition (Jiang et al., 2001).
D2 receptors have an affinity for dopamine 10 to 100 times higher than the D1 receptors (Richfield et al., 1989; Martel and Gatti McArthur, 2020). Affinity of a receptor for a neurotransmitter means the amount (concentration) of the neurotransmitter that is required to activate the receptor. This means that D1 receptors require lots of dopamine to get activated, while D2 receptors can be activated by small concentrations of dopamine. Besides, the low affinity of the D2 receptors means that dopamine can activate them by volume transmission all over the neuron, while the action of the D1 receptors is confined to the synapse. As we will see, this has important consequences for the functioning of the reward pathway and the effect of addictive drugs.
Dopamine receptors in different brain regions
Dopamine receptors are present in different amounts in the brain. Their rank of abundance is D1>D2>D3>D5>D4, so the most common ones are the D1 and D2 receptors (Mishra et al., 2018).
The nucleus accumbens has lots of D1 and D2 receptors and a good amount of D3 receptors (Hurley and Jenner, 2006; Mishra et al., 2018).
The frontal cortex has many D1 receptors, with lower amounts of D2, D3 and D4 receptors.
The cingulate cortex has many D1 receptors and some D4 receptors.
Therefore, the dopamine receptors that interest us are the D1 and the D2 receptors, with D3 and D4 receptors taking the role of the D2 receptors in some brain areas.
D2 receptors in the nucleus accumbens are critical for the functioning of the reward pathway, while D1 receptors in the prefrontal and cingulate cortex mediate the motivation effect of the mesocortical pathway.
The direct and indirect neuronal pathways and their effect on motivation
Another key property of the D2 and D1 receptors is that they are located in different neurons of the nucleus accumbens and the dorsal striatum. These neurons are called medium-sized spiny neurons (MSNs) and release the inhibitory neurotransmitter GABA.
MSNs send axons to other parts of the midbrain, called the basal ganglia, mainly the substantia nigra and the globus pallidus (Hikida et al., 2010; Smith et al., 2013).
MSNs with D1 receptors (D1-MSNs) form the direct pathway, meaning that they make only one synapse inside the basal ganglia (substantia nigra and VTA) before projecting to the thalamus, a way station to the cortex (see figure below).
MSNs with D2 receptors (D2-MSNs) form the indirect pathway, which makes multiple synapses inside the basal ganglia (globus pallidus, then to the substantia nigra and VTA) before sending their message to the thalamus (see figure below).

MSNs also differ in the neuropeptides that they contain (Smith et al., 2013). D1-MSNs express dynorphin, an opioid that activates kappa opioid receptors, and substance P, a neuropeptide that increases pain and stress. D2-MSNs express enkephalin, an opioid that activates mu and delta opioid receptors, and neurotensin (see figure below).

The direct and indirect pathways have key effects on motivation and self-control (Hikida et al., 2010; Kravitz et al., 2012; Trifilieff et al., 2013; Volkow and Morales, 2015).
The direct pathway and its D1 receptors serve to identify stimuli associated with a reward, filtering out stimuli that are not associated with it.
The indirect pathway and its D2 receptors serve to avoid aversive stimuli and to increase motivation to do hard work.
The term reward can be misleading. In the experiments with rodents, rewards are anything that the animal wants, often but not always something pleasurable.
In humans, a reward is a goal we are striving to achieve, regardless of whether it is pleasurable. For example, writing an overlong article about dopamine, or running a marathon. The prefrontal cortex decides what is our goal and then prods the nucleus accumbens to help maintain the effort to achieve it. While the direct pathway maintains the focus on the goal, the indirect pathway keeps us from getting sidetracked by other stimuli, and also provides the motivation for our effort.
Tonic release of dopamine activates D2 receptors
Now that we know about the D1 and D2 dopamine receptors and the direct and indirect pathways, we can explore how they regulate motivation — what I have called mental energy.
In the baseline state of the brain, dopaminergic neurons that go to the nucleus accumbens fire action potentials in a tonic fashion, which means few, widely spaced action potentials. This produces a drip-drip release of dopamine (Wanat et al., 2009; Rice et al., 2011). In this situation, the dopamine levels outside the cell are too low to activate the low-affinity D1 receptors, but high enough to activate the high-affinity D2 receptors (Dreyer et al., 2010; Paladini and Roeper, 2014). This leads to the activation of the D2-MSNs in the indirect pathway, but not the D1-MSNs in the direct pathway. This maintains motivation and effort to complete a task (Salamone and Correa, 2012; Trifilieff et al., 2013; Trifilieff and Martinez, 2014; Volkow and Morales, 2015).
Phasic release of dopamine activates D1 receptors
When there is a strong motivation to get a reward or to achieve a goal, dopaminergic neurons to the nucleus accumbens start firing in bursts of action potentials. Burst firing means groups of high-frequency action potentials separated by short intervals. This induces phasic dopamine release (Wanat et al., 2009; Willuhn et al., 2010; Rice et al., 2011; Wise and Jordan, 2021), meaning short peaks of levels of dopamine high enough to activate the D1 receptors. Importantly, these dopamine peaks are very short, lasting a fraction of a second (Willuhn et al., 2010). However, the peaks happen over and over again, often for a long period time. This recruits the direct pathway, providing a strong push to achieve a goal.
Addictive drugs overstimulate the D1 receptors
In a previous article, Dopamine - Why Heroin Is Addictive But Porn Is Not, I explained how addictive drugs — cocaine, amphetamines, opioids, benzodiazepines, barbiturates, nicotine and alcohol — induce long-lasting increases in dopamine. They do so by either inhibiting (cocaine) or reversing (amphetamines) the dopamine transporter (DAT), by blocking neurons that inhibit dopamine release (opioids, benzodiazepines, barbiturates), or by overstimulating the dopaminergic neurons (nicotine).
While natural stimuli induce short-lasting peaks in dopamine, addictive drugs produce large elevations in dopamine concentrations that last a long time (Grace, 2000). Thus, dopamine peaks produced by natural stimuli typically last a fraction of a second, while drugs increase dopamine levels for minutes (Willuhn et al., 2010).
These high levels of dopamine activate D1 receptors, so clues surrounding the taking of the drug get associated with the D1 receptor activation, producing a memory that guides the person towards taking more drug. This is what generates the drug-seeking behavior.
Addictive drugs downregulate the D2 receptors
However, the effect of these long-lasting increases in dopamine on the D2 receptors is crucial to explain drug addiction. Exposed to too much dopamine for too long, the D2 receptors internalize, disappearing from the cell surface. Repeated drug taking causes the downregulation of the D2 receptors (Porrino et al., 2004; Volkow and Morales, 2015), meaning that the D2 receptor-MSNs of the indirect pathway have less D2 receptors. Receptor downregulation happens when the receptors are internalized too often, so more and more of them get chewed up by proteases instead of being returned to the cell surface. In addition, the D2 receptor gene is expressed less, so fewer D2 receptors are made in the neuron.
The downregulation of the D2 receptors dramatically changes the indirect pathway, leading to a loss of self-control and an increase in impulsivity. So, while the overactivated D1 receptors drive us to seek drugs in the present of drug-taking clues (cocaine lines, bottles of liquor, cigarettes, syringes, etc.), the D2 receptors are not present to reduce our impulsivity and maintain our determination not to take drugs.
All addictive drugs downregulate the D2 receptors, while non-addictive substances do not. There is a strong correlation between the abuse potential of a drug and its ability to downregulate the D2 receptors.
The downregulation of D2 receptors by drugs lasts months. Furthermore, continuous drug use causes the downregulation of the D2 receptors in an expanding volume of the striatum (Porrino et al., 2004).
D2 receptor downregulation is the hallmark of addiction
This way, the reward pathway becomes hijacked by the drug. The direct pathway of the D1-MSNs is taken over to induce drug craving. Meanwhile, the indirect pathway of the D2-MSNs is suppressed, causing a loss in self-control and an increase in impulsivity.
Not only the D2 receptor downregulation is a good predictor of the abuse potential of a drug, but it explains why drug addicts lose the self-control to refrain from seeking the drug, and why they live in a state of dissatisfaction and lack of motivation.
Therefore, the downregulation of the D2 receptors is the hallmark of addiction (Trifilieff et al., 2013; Trifilieff and Martinez, 2014; Volkow and Morales, 2015).
We can use this criterion to determine if behaviors like watching porn, gambling, playing video games and exercising are addictive. If they are, they would downregulate the D2 receptors, like addictive drugs do. I will explore this question in the next articles of this series.
References
Dreyer JK, Herrik KF, Berg RW, Hounsgaard JD (2010) Influence of phasic and tonic dopamine release on receptor activation. J Neurosci 30:14273–14283.
Grace AA (2000) The tonic/phasic model of dopamine system regulation and its implications for understanding alcohol and psychostimulant craving. Addiction 95 Suppl 2:S119–128.
Hikida T, Kimura K, Wada N, Funabiki K, Nakanishi S (2010) Distinct Roles of Synaptic Transmission in Direct and Indirect Striatal Pathways to Reward and Aversive Behavior. Neuron 66:896–907.
Hurley MJ, Jenner P (2006) What has been learnt from study of dopamine receptors in Parkinson's disease? Pharmacol Ther 111:715–728.
Jiang M, Spicher K, Boulay G, Wang Y, Birnbaumer L (2001) Most central nervous system D2 dopamine receptors are coupled to their effectors by Go. Proceedings of the National Academy of Sciences 98:3577–3582.
Kravitz AV, Tye LD, Kreitzer AC (2012) Distinct roles for direct and indirect pathway striatal neurons in reinforcement. Nature Neuroscience 15:816–818.
Martel JC, Gatti McArthur S (2020) Dopamine Receptor Subtypes, Physiology and Pharmacology: New Ligands and Concepts in Schizophrenia. Frontiers in pharmacology Volume 11 - 2020.
Mishra A, Singh S, Shukla S (2018) Physiological and Functional Basis of Dopamine Receptors and Their Role in Neurogenesis: Possible Implication for Parkinson's disease. J Exp Neurosci 12:1179069518779829.
Paladini CA, Roeper J (2014) Generating bursts (and pauses) in the dopamine midbrain neurons. Neuroscience 282:109–121.
Porrino LJ, Lyons D, Smith HR, Daunais JB, Nader MA (2004) Cocaine self-administration produces a progressive involvement of limbic, association, and sensorimotor striatal domains. J Neurosci 24:3554–3562.
Rice ME, Patel JC, Cragg SJ (2011) Dopamine release in the basal ganglia. Neuroscience 198:112–137.
Richfield EK, Penney JB, Young AB (1989) Anatomical and affinity state comparisons between dopamine D1 and D2 receptors in the rat central nervous system. Neuroscience 30:767–777.
Salamone JD, Correa M (2012) The mysterious motivational functions of mesolimbic dopamine. Neuron 76:470–485.
Smith RJ, Lobo MK, Spencer S, Kalivas PW (2013) Cocaine-induced adaptations in D1 and D2 accumbens projection neurons (a dichotomy not necessarily synonymous with direct and indirect pathways). Current Opinion in Neurobiology 23:546–552.
Trifilieff P, Martinez D (2014) Imaging addiction: D2 receptors and dopamine signaling in the striatum as biomarkers for impulsivity. Neuropharmacology 76 Pt B:498–509.
Trifilieff P, Feng B, Urizar E, Winiger V, Ward RD, Taylor KM, Martinez D, Moore H, Balsam PD, Simpson EH, Javitch JA (2013) Increasing dopamine D2 receptor expression in the adult nucleus accumbens enhances motivation. Molecular Psychiatry 18:1025–1033.
Volkow ND, Morales M (2015) The Brain on Drugs: From Reward to Addiction. Cell 162:712–725.
Wanat MJ, Willuhn I, Clark JJ, Phillips PE (2009) Phasic dopamine release in appetitive behaviors and drug addiction. Curr Drug Abuse Rev 2:195–213.
Willuhn I, Wanat MJ, Clark JJ, Phillips PE (2010) Dopamine signaling in the nucleus accumbens of animals self-administering drugs of abuse. Curr Top Behav Neurosci 3:29–71.
Wise RA, Jordan CJ (2021) Dopamine, behavior, and addiction. J Biomed Sci 28:83.
Copyright 2025 Hermes Solenzol


Comments